Outsourcing activity remains strong and unlikely to abate, especially in more traditional areas.
Over the past 11 years, BioPlan’s annual report and survey of biopharmaceutical manufacturing has noted a shift in outsourcing to a more strategic calculus involving activities some had previously considered too core to outsource. At the same time, data have, not surprisingly, illustrated that outsourcing tends to be dominated by lower-value activities, these being the easiest for facilities to gain comfort in handing off to partners. So essentially, this year BioPlan found something of a bimodal trend, more complex process outsourcing and greater outsourcing volume of basic bioprocessing, such as fill-finish, and other activities.
With the release of the 11th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production,BioPlan reviews the responses from nearly 250 biopharmaceutical manufacturers and their perspective on, among many other aspects in bioprocessing, the landscape of outsourcing activity. The sample size is large enough to shape a view of the top activities being outsourced and what this year might hold.
Most commonly outsourced activities
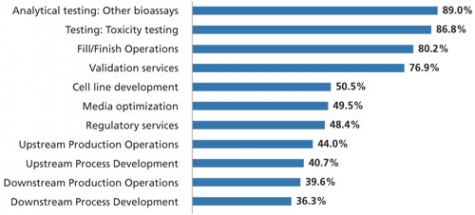
Results from BioPlan’s 2014 study’s preliminary data indicate that—as with the past two years—the most commonly outsourced activity is analytical testing of bioassays, by about 9 in 10 respondents to date. Following that is toxicity testing, which is being outsourced to some extent by 86.8% of respondents so far. Fill/finish operations are also being outsourced by more than 8 in 10 respondents (80.2%), while validation services (76.9%) rounds out the top four.
On the other end of the spectrum, design of experiments (DoE) (34.1%), downstream process development (36.3%), and quality-by-design (QbD) services (37.4%) are the least likely to be outsourced, although at minimum about one-third of the industry appears to be outsourcing at least some degree of these activities.
If current trends hold, these results would in some cases mark a significant departure from 2013 rankings (1). Relative to other activities, for example, this year’s results suggest that toxicity testing and fill/finish operations are moving up the outsourcing popularity ranks (see Figure 1).
 utsourcing less common activities. We find that, of the top six activities being outsourced this year, four are being outsourced to greater degrees than last year. Virtually all of the others, however, are being outsourced by fewer biopharma respondents this year.
utsourcing less common activities. We find that, of the top six activities being outsourced this year, four are being outsourced to greater degrees than last year. Virtually all of the others, however, are being outsourced by fewer biopharma respondents this year.