June 29, 2014
Concentrating Feed—an Applicable Approach to Improve Antibody Production
By: Ping Xu, Xiao-Ping Dai, Albert Kao, Rosario Scott, Reb Russell BioPharm International
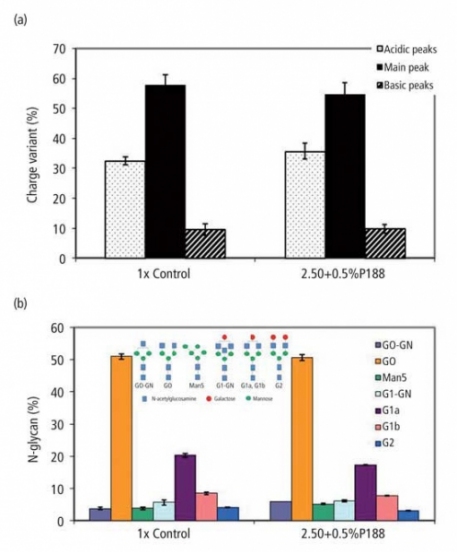
Antibody production in 7-L bioreactors using concentrated feed media of M1. To test the scalability as well as to remove the negative effects from the high pH feed media, 7-L bioreactors were used to run cell line A process with pH control. The profiles of viable cell density and viability are shown in Figure 2a and 2b. The 2.50X + 0.5% P188 medium was tested in parallel to the 1X medium that was used as a control. The viability for the control decreased from day 8, whereas the concentrated feed maintained higher viability for a longer period of time. The productivity profiles shown in Figure 2c indicate that use of the concentrated feed medium improved the productivity by 33% towards the end of the process. The use of the concentrated feed medium also led to a significantly higher (p < 0.05) specific productivity than the control (data not shown). These results suggest that productivity can be improved when feed volume is reduced, which is consistent with the shake-flask studies. The product qualities, including charge heterogeneity and N-glycan profiling, were not affected by the increase of P188 concentration in the feed (see Figure 3). There were no changes in antibody monomer percentages and protein purity with addition of P188 (data not shown).
Antibody production in 5000-L bioreactor using concentrated feed media of M2. The concept of concentrated media was applied in the development of a feed medium for the process of cell line C. A feed medium was developed by concentrating the original feed medium to 7.5 fold, designated as M2 (see Table I). From M2, a 10-fold increase of the original feed medium was achieved by adjusting pH to between 8.8 and 9.5, leading to 1.33X M2 feed medium. The two media were tested in parallel using 7-L bioreactors. The feeding volume of 1.33X M2 was reduced by 33% so that the total amount of each component added to the bioreactor was the same as that of the 1X M2 control. Data from a 5000-L manufacturing bioreactor run performed using 1.33X M2 feed medium are overlaid in Figure 2d. The productivity of 1.33X M2 was 11% higher than that of 1X M2 at both development and 5000-L manufacturing scales.
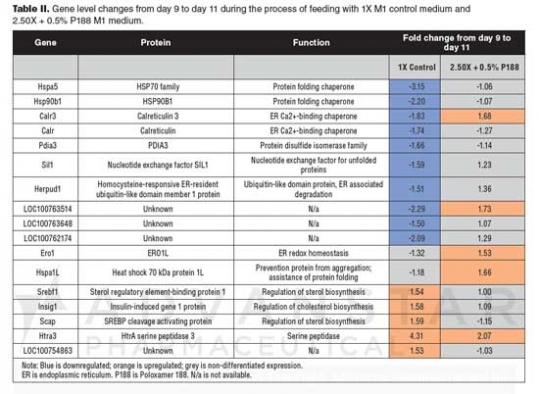
Gene regulation by feeding with concentrated media. To understand the rationale for improving antibody specific productivities in cultures fed with concentrated feed media (see Figure 1), an unfolded protein response qPCR array was chosen in this study. The array included 84 genes for protein expression, folding, and secretion. Gene level changes from day 9 to day 11 in the protein-production phase were investigated in this assay, using two samples prepared on both days from 1X M1 feed medium treatment and two samples from 2.50X + 0.5% P188 treatment. Gene levels that changed more than 1.5-fold from these treatments are listed in Table II. The downregulated genes in the control samples (highlighted in blue) include heat shock protein family genes (Hspa5, Hsp90b1), Ca2+-binding chaperones (Calr3, Calr), protein disulfide isomerase family gene (Pdia3), nucleotide exchange factor for unfolded proteins (Sil1), ubiquitin-like domain protein for endoplasmic reticulum (ER)-associated degradation (Herpud1), and three unknown genes. Instead of downregulation, these genes were either upregulated or non-differentially expressed in cells fed with 2.50X + 0.5% P188 (see Table II). Highlighted in gray in Table II, Ero1 encoding an ER oxidoreductase and Hspa1L encoding an ER chaperone were non-differentially expressed in cells fed with the 1X medium, but up-regulated in cells fed with 2.50X + 0.5% P188. These data suggest that the 2.50X + 0.5% P188 medium could maintain and elevate levels of genes for chaperones and folding enzymes that ensure protein quality control in the secretory pathway. This finding is consistent with the higher cell viability and higher productivity observed in the concentrated medium.

