August 23, 2013
Essentials in Quality Risk Management
Thomas A. Little, PhD, Biopharm International Magazine
Quality risk management is an essential element of every aspect of drug development and manufacturing throughout the product lifecycle.
Quality risk management (QRM), as defined in International Conference on Harmonization's (ICH) Q9 document (1), is designed to ensure that drug critical quality attributes (CQAs) are defined and maintained from phase to phase during drug development and manufacturing and changes in drug-product formulation, definition, analytical method, and associated process changes are understood and managed to ensure patient safety and drug efficacy. An effective QRM process can further ensure the high quality of the drug product to the patient by providing a proactive means to identify and control potential risks to quality during development and manufacturing.
Risk can be defined two ways. First, as the combination of the probability of occurrence of harm and the severity of that harm, or second, as the potential influence of product and process factors on CQAs and the uncertainty of that influence. The first definition is traditional and takes into account potential failures and adverse events. The second takes into account influence of factors relative to CQAs and how well we understand or know the influence of those factors on the CQAs of the drug product. In modern drug development, it is often the second definition that is a problem in that we don't know what we don't know. As we complete development activities, the risk goes down because our knowledge and understanding of the factors associated with unit operations and analytical methods go up relative to product acceptance and all associated CQAs.
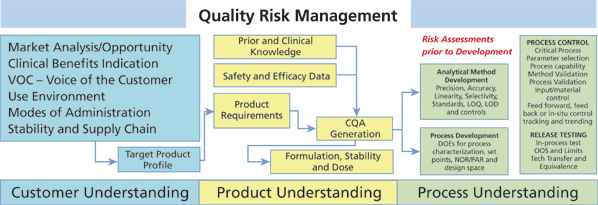
There are two key principles in QRM. First, risk assessment should be based on scientific knowledge associated with product and process understanding, and second, the level of effort associated with risk assessment/management should be commensurate with the level of risk being identified and evaluated. Science and data help to minimize and control risk. A common criticism of risk assessments is they are just opinions and not backed by science. The higher the focus and dependence on scientific principles, thought experiments, literature/research, prior experimental results, and understanding of historical performance, the better the science brought into the QRM process. The major benefits of QRM and risk assessments are to improve the ability to develop drug products and drug substance and answer the question, "When, what, how much, and where do we need additional development?" Development activities reduce potential risks to safety and efficacy and help to achieve the benefits of the drug for the targeted indication.
Systematic drug development and QRM (see Figure 1) ensure that we maintain line of site from the target product profile, to the quality target product profile (QTTP), through CQA generation to analytical method selection, unit operation characterization, specification and acceptance criteria limit generation, critical process parameters and control selection, and finally, method and process validation (see Figure 2). ICH Q6, Q8, Q9, Q10, and Q11 detail the pharmaceutical development process and how to set limits (1-5). The QRM process is linked to product and process CQAs because they are the quality attributes of the drug.