Use of multiple washes in Protein A
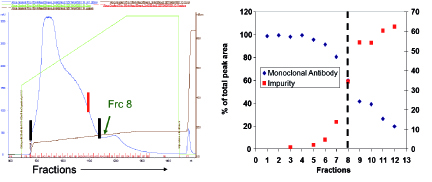
To understand the resolution of fragments and aggregates on Protein A, a pH gradient elution between pH 5.0 and 3.0 over 10 column volumes was carried out, and fractions were collected for analysis via high-performance size exclusion chromatography (HPSEC). Based on the data presented in Figure 2, resolution between low molecular weight fragments and monomeric antibody could be achieved. These results led to inclusion of a two-step elution strategy where a high pH (4-5) wash followed by low pH (3-4) elution was used to minimize the fragment content in the Protein A product pool. When implemented for a specific product, the revised elution scheme led to a 5% absolute reduction in fragment content with a 10% yield loss.
Gradient elution for cation exchange chromatography
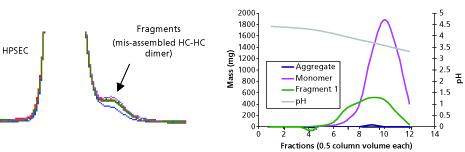
Another modification to the traditional antibody purification platform is the inclusion of gradient elution during cation exchange chromatography (CEX). Typically, the elution for CEX is isocratic (constant salt and pH conditions). Under certain cases, however, this may lead to issues of robustness and other complications (4). During development of therapeutic candidates expressed in dhfr- CHO, for example, an impurity that was later identified as the mAb with unprocessed heavy chain leader sequence was insufficiently cleared during the CEX step. For removal of this impurity, operating conditions needed to be modified to incorporate lower loading than normally established within platform and to include a salt gradient elution methodology. Figure 3 shows the elution and pool cutting strategy for removal of this impurity. With these modifications, 90% removal of the impurity could be achieved.
Another modification to the traditional antibody purification platform is the inclusion of gradient elution during cation exchange chromatography (CEX). Typically, the elution for CEX is isocratic (constant salt and pH conditions). Under certain cases, however, this may lead to issues of robustness and other complications (4). During development of therapeutic candidates expressed in dhfr- CHO, for example, an impurity that was later identified as the mAb with unprocessed heavy chain leader sequence was insufficiently cleared during the CEX step. For removal of this impurity, operating conditions needed to be modified to incorporate lower loading than normally established within platform and to include a salt gradient elution methodology. Figure 3 shows the elution and pool cutting strategy for removal of this impurity. With these modifications, 90% removal of the impurity could be achieved.