The authors review how media components modulate the quality of monoclonal antibody products.
By Anurag Rathore, Rajinder Kaur, Dipankar Borgayari

Recombinant protein products have made a revolutionary impact on human healthcare by enabling mass production of safe and effective therapeutic drugs such as hormones, growth factors, blood coagulation products, thrombolytic agents, vaccines, and monoclonal antibodies (mAbs). Of these, mAbs comprise large, multi-domain proteins that require complex machinery for proper protein folding, assembly, and post-translation modifications. These modifications can profoundly affect protein quality relevant to clinical efficacy, safety, and half-life of biologics (1). Mammalian cells meet these criteria quite efficiently by virtue of their post-translation modification system, and this has made them a dominant production system for the biopharmaceutical industry. Major mammalian expression systems for the production of biotherapeutics include NS0 cells, baby hamster kidney (BHK) cells, hybridoma cells, PER.C6 cells, human embryonic kidney (HEK) cells, and Chinese hamster ovary (CHO) cells (2).
The cell-culture process and media are known to have a significant effect on the expression and stability of therapeutic products as well as their critical quality attributes (CQAs) including glycosylation, sialyation, charge variants, and aggregation among others (1). Optimal growth of cells requires monitoring and control of process variables such as pH, dissolved oxygen, dissolved carbon dioxide, and temperature. Arguably the most important and -crucial step in cell culture, however, is the selection of appropriate growth medium for in vitro cultivation. The cell-culture medium provides an artificial environment conducive to survival and proliferation of cultured cells and maintains the desired pH and osmolality. It is typically composed of a complex mixture of amino acids, vitamins, inorganic salts, glucose, and serum as a source of growth factors and hormones. The choice of cell culture media has been known to significantly affect the physiochemical characteristics of mAbs. The selection of the media depends on the type of cells to be cultured and also the purpose of the culture and resources. Different cell types have highly specific growth requirements; therefore, the most suitable media for each cell type has to be determined experimentally. The complexity of composition of cell-culture media offers many challenges towards optimization of the individual media components (2–3).
The formulation of optimal culture media for propagation of cultured mammalian cells has received a lot of interest for many years as a result of its critical impact on the animal cell technology based bioprocess development (3). Factors that play a role in media design originate from three major sources, namely the attributes of the product under consideration, the cell line that is being used for protein production, and the manufacturing process (i.e., fed batch, perfusion, or continuous). Different cell lines have different nutritional requirements because of the differences in metabolism, and this factor needs to be appropriately accounted for during media optimization. While natural media consists solely of naturally occurring biological fluids and has been proven to be quite useful and convenient for a wide range of animal cell cultures, a key disadvantage is the possibility of poor reproducibility originating from the lack of knowledge of the exact composition (4). Synthetic or chemically defined media are prepared by adding defined concentration of nutrients (both organic and inorganic) such as vitamins, salts, trace elements, carbohydrates, and cofactors. Establishing the role of numerous media components and supplements upon product quantity and quality is of paramount importance in media optimization with a focus upon reduced production of undesired metabolites. A deeper insight in understanding of metabolism and cell mechanisms is required to define strategies for providing effective means to reduce by-products and increase productivity d quality of biologics.
CHO cells are the predominant production hosts for recombinant protein production due to their adaptability to various culture conditions and plasticity with reference to genetic alterations (2–5). Intense efforts in bioprocess development have been made to improve the production of CHO-based biopharmaceuticals by cell line engineering, improved methods for cell growth, and upregulating the post-translational modifications and enhanced protein stability through targeted approaches at genomic and cellular level (6–8). Cell-culture media has been known to significantly impact cell culture performance, downstream processing, as well as product quality (1, 3). While substantial progress has been achieved in this field, it may best be described as conservative and pragmatic. To date, the function of many components of the growth medium essential for cellular proliferation and biological production and quality of biotherapeutics has not been precisely defined at the molecular level.
In this 37th article in the “Elements of Biopharmaceutical Production” series, the authors review the topic of how media components modulate the quality of recombinant protein products and explore a relationship between media design and CQAs.
Media design for gaining higher productivity and modulating recombinant protein product quality
Formulation of cell-culture media is an important activity under process development. Manufacturers spend extensive effort and time on this activity in absence of any generic procedure (6, 7). Traditional optimization methods, such as the titration of single components or “one-factor-at-a-time” approach, are useful, but can be labor-intensive and time-consuming. Screening experimental designs with these components at low and high ranges of concentration is helpful for determining which components have important effects on productivity and quality (9). Design-of-experiments (DoE) methods can be useful in efficient experimental examination of these factors. During this activity, changes in the osmolality of the medium and generation of metabolic waste products are carefully monitored (8-10). In the following, the authors highlight some of the key findings that relate the impact of media components upon CQAs of recombinant therapeutic products.
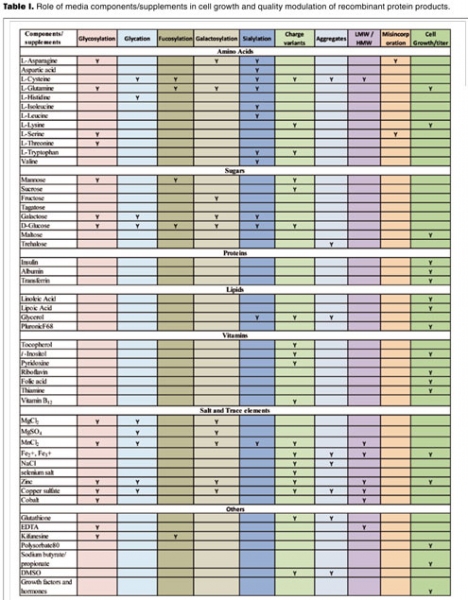
Figure 1 and Table I present relevant information on this topic.
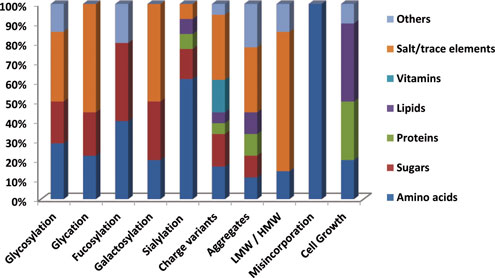
Amino acids
One of the basic nutrients, amino acids, are known to promote cell growth and productivity. They are a source of nitrogen and building blocks for proteins as well as mediators of numerous metabolic pathways. Amino acid supplementation is recognized as one of the vital components in cell-culture medium design and optimization (5). During CHO batch shake flask culture, lower fucosylation occurs with decreasing glutamine concentration (0-8 mM) and it is assumed that the reduction in the glycolytic flux due to glutamine limitations affects glycosylation. Asparagine concentration has been reported to affect galactosylation level.
Cysteine, along with temperature shift, is believed to reduce protein aggregates and increase their stability in culture harvests. The oxidized form of cysteine has been shown to reduce the formation of high-molecular weight (HMW) forms and also result in greater sialyation and higher harvest titer (10). Glutamine (Gln) concentrations below 1 mM are reported to be detrimental to glycosylation or, if desired, to allow the production of non-glycosylated molecules. CHO cells in fed-batch mode at low glutamine (<0.1 mM) concentrations have been shown to result in reduced sialylation and increased presence of minor glycan species consisting of hybrid and high-mannose types (11). Histidine supplementation or basic residues seem to favor glycation in structurally known proteins. The oxidized form of cysteine also reduces HMW and fragmentation. During cell culture, starvation of asparagine has been shown to result in misincorporation of serine during translation (11). Serine has been reported as a growth-limiting nutrient used in batch culture of CHO cells. During hybridoma cultivation, amino acids such as glutamine, asparagine, and glycine are known to prevent cellular apoptosis. Balanced supplementation of amino acids has been shown to increase viable cell density and final concentration of recombinant antibody (10–12).
Carrier proteins
Carrier proteins act as a carrier or transporter to a variety of substances including lipids, trace elements, amino acids, and vitamins. As lipids cannot dissolve in an aqueous solution by themselves, they are more effectively supplied to cells after forming complexes with albumin. In addition, albumin has toxin-neutralizing, antioxidant, and shear stress-reducing effects (2). Transferrin is used as a carrier of iron. Lactoferrin serves as a substitute for transferrin. Albumin in combination with transferrin improves cell growth in majority of mammalian cells. It is also reported to be used as a supplement for enhanced cell growth and expression of mAbs (13).
Growth factors
Growth factors are added in extremely low concentrations and are known to induce proliferation, differentiation, secretion, or import. Many cells require supplementation of the medium with growth factors under serum-free conditions. Acidic fibroblast growth factor (FGF) readily degrades if heparin sulfate is not present on the surface of the target cells; therefore, heparin or synthetic dextran as a substitute is added to the medium in some situations (2). Other growth factors used as supplement are epidermal growth factor, insulin growth factor, and TGF-β. Insulin growth factor shows positive effect on cell growth as well as antibody titer (5).
Hormones
Hormones enhance cell growth by activating signal transduction pathways associated with cell differentiation in a variety of cells including CHO. Hydrocortisone improves the cloning efficiency of the glial cells and fibroblasts (2). Along with hydrocortisone and prolactin, various combinations of estrogen, androgens, and progesterone are necessary for the maintenance of the mammary cell culture. Hydrocortisone is related to cell proliferation in various animal cell cultures. Maintaining high viable CHO cell density with tri-iodothyronine resulted in higher antibody concentrations (5).
Lipids
Lipids as a membrane component play a role in transport and signal transduction. Lipid supplementation is known to affect cell growth and protein expression in different cell lines.
It has been shown that nanoparticle cholesterol mixtures enhance mAb expression. Various lipids are used as supplements including cholesterol, steroids, fatty acids (e.g., palmitate, stearate, oleate, linoleate), ethanolamine, choline, and inositol (2, 10).
Polyamines
Polyamines are low-molecular-weight, basic, physiologically active amines that exist ubiquitously in cells and promote protein or nucleic-acid synthesis. Polyamines such as putrescine, spermidine, and spermine play key role in orchestrating cell growth and apoptosis (2).
Protein hydrolysates
The addition of animal-component free protein hydrolysates, as a substitute for serum, is used to increase cell density, culture viability, and productivity in an efficient manner. Protein hydrolysates are composed of amino acids, small peptides, carbohydrates, vitamins, and minerals, which provide nutrient supplements to the medium (2,7). Non-animal derived hydrolysates from soy, wheat, and yeast are commonly used in cell culture media.
Protective agents and detergents
These chemicals are typically used to reduce the shear stress generated in stirred cultures and by pipette manipulation. Pluronic F-68 and Tween 80 are also commonly used as solubilizers of lipophilic substances such as lipids and fat-soluble vitamins. Pluronic F-68 and Putrescine showed positive effect on cell growth and antibody production (2). Carboxymethyl cellulose and polyvinyl pyrrolidone are used as protective agents. Dimethyl sulfoxide (DMSO) and glycerol also exhibit protein stabilization capabilities. DMSO concentrations from 1% to 8% (v/v) have been known to reduce sialylation but also negatively impacted cell proliferation (5, 7).
Reductants
Reductants are necessary to maintain intracellular redox environment for correct folding and functioning of various proteins. Import of cystine or cysteine into cells is necessary to maintain such environment by addition of reducing agents to the culture medium. Reductants used in cell-culture medium are 2-mercaptoethanol, α-thioglycerol, and reduced glutathione (2). Glutathione has been proved to reduce protein aggregation. Glutathione, a tri-peptide, protects cell from oxidative stress and plays an important role in cell viability and growth (5, 10).
Salts
Inorganic salts in the media retain the osmotic balance and help in regulating membrane potential. During CHO batch culture, aggregation in the presence of NaCl (40 mM) decreases from 86% to 62% and completely vanishes when the concentration is increased to 85 mM compared to the control cultures. Copper sulphate has been also proven to reduce aggregation. Sodium citrate acts as critical component for continuous cell growth and upregulated recombinant protein production (7–10).
Sugars
Sugars are a major source of energy for the cells and also help in maintaining osmotic balance. The addition of sucrose has been known to result in higher levels of high mannose glycans. Glucose (< 0.70 mM) concentrations resulted in decreased sialylation and increased presence of minor glycan species (10). Supplementation of culture medium with galactose increased glycosylation.
The limitation of glucose in the culture medium allowed reduction in lactate production whereas critical limitation of the former resulted in glycosylation heterogeneity due to reduced availability of uridine diphosphate-acetylglucosamine (UDP-GlcNAc). Glucose concentrations below 1 mM have been reported to be detrimental to glycosylation (11-13). The presence of hexoses in the culture medium including the frequently used glucose and galactose may lead to glycation. Supplementation of sucrose and tagatose led to targeted manipulation of protein glycosylation profiles from cultured mammalian cell lines. These sugars played an effective role in redistributing the N-glycan glycoform profile towards high mannose species, thus reducing overall fucosylated species.
Mannitol, maltose, and glucose have been shown to protect residues from oxidation by acting as chelating agents that remove catalyzing metals and thereby reduce the oxidative stress. It was observed that addition of 150-200 mM trehalose to the medium of a CHO cell culture prevented polymerization and aggregation of the recombinant protein (2, 13). Supplementation of serum-free media with maltose resulted in sustained cell growth and improved recombinant protein production in batch shake flask cultures (14).
Transition metals
These elements readily undergo electron transfer and function in the active centers of enzymes and physiologically active substances inside the cell. Transition metals such as manganese are known to increase sialyation in the presence of uridine and galactose (10). Copper (16-22 µM) increases glycation whereas it is reciprocally regulated by zinc (10-16 µM) and magnesium (0.62-0.93 mM) and also reduces formation of HMW species. The presence of transition metal ions such as iron (Fe2+, Fe3+) and copper (Cu2+) favor oxidation of amino-acid residues in proteins.
With the increase in Fe content, deamidation increases, and reduced formation of aggregates is observed. Increased copper concentration and decreased zinc level result in enhanced formation of lysine variants (12). Supplementation of culture medium with iron and zinc significantly enhanced mAb and rIFN-β production, respectively (14, 15). Manganese, as a cofactor of many enzymes, modulates the glycosylation profile of many proteins by regulating enzymes of glycosylation pathway. However, it increases the level of high mannoses in glucose-limiting or glucose-absent conditions. It has also been known to play an important role in determining levels of the various oligosaccharide species including high mannose, galactosylated, fucosylated, and sialylated proteins. The addition of copper has been reported to decrease formation of HMW species in mAbs expressed in CHO cells. Cultures performed at higher Mn2+ concentrations (40 µM) exhibited increased galactosylation and higher sialylation of recombinant human erythropoietin. Culture medium supplemented with Cu2+, Zn2+, Mg2+resulted in formation of glycated proteins (10). Supplementation of media with iron plays a major role in the generation of glycated-protein products. A study recognized the excellent inhibitory effect of Mn2+ and the stimulating effect of Zn2+ upon formation of advanced glycation end (AGE) products (10).
Supplementation of CHO cell cultures with Mn2+ are reported to increase site-occupancy and galactosylation of various recombinant glycoproteins. The presence of transition metal ions such Fe2+, Fe3+ and Cu2+ favors oxidation of amino-acid residues in proteins. Formation of basic variants of mAbs has been linked to copper concentration in CHO fed-batch cultures (4). Copper concentration in the cell culture medium has been reported to be the most significant parameter affecting formation of the C-terminal Lys variants. Iron concentration has been shown to significantly affect formation of acidic variants of mAbs. Iron plays a major role in inhibiting the formation of aggregates whereas the abundance of HMW species was significantly lower at low iron concentrations (0-4 ppm) in the presence of a chelator (5–7). Higher Cu2+ ion concentration resulted in the higher level of proline amidation. Increase in copper and a decrease in zinc concentrations in cell-culture medium increased levels of C-terminal Lys in a recombinant mAb. Levels of Zn/Cu/Se ions in culture medium have been reported to have an effect on mAb fragmentation (10–13).
Vitamins
Vitamins are crucial for cell division and growth and act as precursors of various cofactors; vitamins are known to play a major role as antioxidants. Water-soluble vitamin E (tocopherol) and vitamin B6 (pyridoxine) have antioxidant properties and reduce amino acid oxidation due to their free-radical-scavenging capabilities. Various B-vitamins significantly affect formation of acidic variants of mAbs. Hypoxanthine and thymidine are able to stimulate initial cell growth (2, 7).
Others
Kifunensine, an alkaloid, has been known to affect glycosylation by regulating enzymes involved in the process. It is effective in producing antibodies with oligomannose-type glycans from CHO cells (2, 10). Gallic acid and its derivatives efficiently inhibit fucosylation in the presence of 10-15 mM Mn2+. Poloxamer 407 (Pluronic F127) at concentrations >17% (w/w) hampered the rate of deamidation up to 40%. Polyols at high concentrations displayed a protective effect on oxidation. Addition of 0.01% (v/v) Polysorbate 80 into chemically defined concentrated feed media reduced overall aggregation levels by 2.6-2.7% in two different CHO cell lines (10). Solvent conditions (pH, temperature) and the presence of metals or radicals play important role in formation of low molecular weight species of mAbs.
EDTA inhibits fragmentation of mAbs (12). Thioredoxin (Trx) activity diminishes in the presence of 50-100 µM copper sulfate (CuSO4), which consequently minimizes free thiols and subsequently fragmentation. Culture medium supplemented with β-mercaptoethanol and chloroquine, specific antibody productivity has been reported to be relatively high compared to basal medium (5). Sodium butyrate and sodium propionate, an alkaloid have been reported to enhance mAb production manifolds (14–16).
Future Perspective
Progress in cell-culture technology has led to substantial enhancement in production of recombinant protein products mostly due to robust vector design and host cell engineering methods. However, media development continues to remain a key activity that is not completely understood. Synergistic approach of using selective combination of supplements at specific times of addition holds potential to improve recombinant protein productivity.
Exploration and experimentation in the area of media development is needed along with mechanistic understanding of how they impact the process and the product. A rationally designed media will subsidize our knowledge of cellular metabolomics and its modulation for development of recombinant protein products with desired product titer and customized therapeutic activity.
References
1. A. S. Rathore, Trends in Biotechnology 27: 698-705 (2009).
2. Tatsuma Yao and Yuta Asayama, Reprod Med Biol. 16: 99-117 (2017).
3. Jee Yon Kim et al., Appl Microbiol Biotechnol 93: 917-930 (2012).
4. James W. Brooks et al., BioPharm International 29 (11) (2016).
5. Do Yun Kim et al., Biotechnology Prog. 47: 37-49 (2005).
6. A. S. Rathore et al., Biotechnology Prog. 31: 1586-99 (2015).
7. Fatemeh Torkashvand and Behrouz Vaziri, Iran Biomed J. 21: 131-41 (2017).
8. M. Zhang and T Hill, J Bioanal Biomed. 8: 23-27 (2016).
9. Ananth Parampalli et al., Cytotechnology 54: 57-68 (2007).
10. D. Bruhlmann et al., Biotechnol. Prog. 31: 615-29 (2015).
11. Fatemeh Torkashvand et al., PLOS ONE 10: 1-21 (2015).
12. Kaschak T. et al., mAbs 3: 577-83 (2011).
13. P. Hossler et al., Biotechnol. Prog. 30: 1419-31 (2014).
14. Dawn Sow Zong Leong et al., “Evaluation and use of disaccharides as energy source in protein-free mammalian cell cultures” SCIENTIFIC REPORTS (2017) DOI: 10.1038/srep45216
15. R. Zuqueli et al., Lat. Am. appl. res. 36: 321-27 (2006).
16. Bai Y et al., Biotechnol Prog. 271: 209-19 (2011).