The authors review efforts to limit polymer degradation without significantly impeding cell growth.
Jan 01, 2015
By Sara Ullsten, Shujian Yi, Jeffrey Carter, Eva Lindskog, Pokon Ganguli, Hernan Parma, Eva Blanck
This article discusses efforts to limit polymer degradation without significantly impeding cell growth. The authors were able to show that a single resin can be converted to films that lead to substantially different amounts of leached degradation products, depending on how the resin is processed.
 The use of disposable equipment in the biopharmaceutical industry is rapidly increasing, as it offers advantages in terms of flexibility, elimination of cleaning procedures, and reduced capital expenditure (1). The materials used to fabricate single-use equipment are primarily polymeric. The chemical and physical properties of polymeric materials will be influenced by molecular structure, polymerization process, stabilization and processing additives, as well as manufacturing factors such as extrusion conditions. Further, the physicochemical properties of a polymeric material may also be affected by external factors such as heat, light, oxygen, and sterilization conditions.
The use of disposable equipment in the biopharmaceutical industry is rapidly increasing, as it offers advantages in terms of flexibility, elimination of cleaning procedures, and reduced capital expenditure (1). The materials used to fabricate single-use equipment are primarily polymeric. The chemical and physical properties of polymeric materials will be influenced by molecular structure, polymerization process, stabilization and processing additives, as well as manufacturing factors such as extrusion conditions. Further, the physicochemical properties of a polymeric material may also be affected by external factors such as heat, light, oxygen, and sterilization conditions.
Disposable bioprocessing containers, such as those used for fluid storage or as bioreactor bags, are usually made from multilayer films. Typical resins used in multilayer films include, but are not limited to, variants of polyethylene (PE) (e.g., low-density polyethylene [LDPE], linear low-density polyethylene [LLDPE], ultralow-density polyethylene [ULDPE], ethylene vinyl acetate [EVA], ethylene vinyl alcohol [EVOH], polyamide [PA], or polyethylene terephthalate [PET]). In such films, the attributes of each layer contribute to the overall properties of the film, and resin selection is based on criteria set for, for example, barrier properties or transparency requirements. In some cases, the different polymer layers in a multilayer structure show limited interlayer adhesion (2). In such cases, tie layers are used to provide adhesion between adjacent film layers of the selected resins (3).
To achieve and maintain the desired property of a polymeric material, to protect it from degradation, and to enhance its properties or processing characteristics, a range of additives is available (4). Examples include antioxidants that are added as polymer stabilizers. Stabilizers protect polymers from the heat and shear encountered during extrusion and offer protection throughout the shelf life of the material. A broad range of different antioxidants is available; these antioxidants all have different working mechanisms to prevent or limit polymer degradation. Especially in polymer-melt processing, organophosphite compounds are widely used to ensure decomposition of hydrogen peroxide species. In blends with sterically hindered phenols, organophosphite compounds represent the most widely used antioxidant package for polymers. Global consumption of phenolic and organophosphite antioxidants represent approximately 85% of all global antioxidant consumption in plastics (5).
For films used in single-use containers, one of the major sources of degradation is sterilization. Ionizing radiation (gamma rays or electron beams), commonly used to sterilize single-use biomanufacturing equipment, can induce chemical and physical changes in the film, such as chain scission or crosslinking. Radiation degradation, UV degradation, and thermo-oxidative degradation are all similar chain reactions, and thus, a single polymer stabilization system can be used to prevent initiation of degradation reactions and/or stop propagations induced by all three degradation triggers (6). The stabilization system used is typically composed of a mixture of primary and secondary antioxidants that exhibit a synergistic effect (7). It is important to carefully select a sound stabilization system not only from a physical point of view, but also from a chemical and toxicological perspective, as degradation products of stabilizers will be part of the leachables and extractables profile of a material. A recent reported example showed that the oxidized form of the organophosphite secondary antioxidant tris(2,4-di-tert-butylphenyl)phosphite (TBPP, often referred to under the trade name Irgafos 168) undergoes further degradation upon gamma irradiation (8). One of the degradation products, bis(2,4-di-tert-butylphenyl)phosphate (bDtBPP), inhibits growth of some Chinese hamster ovary (CHO) cell lines (8, 9, 10).
A stabilizer-degradation product impacting cell growth is a concern in single-use systems (SUS). Options for eliminating the detrimental effects of bDtBPP include complete removal of TBPP from the formulation, controlling TBPP oxidation through modulation of film manufacturing parameters, or using a different antioxidant. An additional option is to alter the gamma radiation level or conditions, but this may not be feasible due to minimum requirements for sterilization or process capability. There is no singularly correct approach. Removal of the antioxidant could lead to poor film properties, film manufacturing settings might not be adjustable to minimize oxidative conditions, and alternative antioxidants (and their degradation products) are not as well characterized as those of TBPP. Complete removal of TBPP is not necessary, as many single-use bags containing TBPP perform well in cell-culture applications (11). By optimizing the level of TBPP in the formulation, both polymer degradation protection and good cell growth performance can be achieved (12). The present case study shows that film-manufacturing parameters can be controlled to manufacture, from a cell-culture perspective, high-quality and low-quality films.
Materials and Methods
Resins, films, and standards of TBPP used in the study were kindly provided by Renolit, in Enkhuizen, The Netherlands. This included Solmed Infuflex 9101 resin and film (denoted A), PE prototype resin (denoted B), and prototype films (denoted A’ and B). Film samples were selected from standard production conditions or were prototypes manufactured explicitly for this case study. Cellbag culture chambers (containers) were manufactured at a Cytiva pilot manufacturing facility in Westborough, MA, US. Gamma irradiation of containers was performed by Steris Isomedix, Northborough, MA, US at a dose range of 27.5-40 kGy using a 60Co source. The compound bDtBPP (98% purity, as determined by HPLC analysis) was provided by Amgen Corp., CA, US. In this study, the monoclonal antibody-expressing CHO DG44 cell line was used under license from Cellca GmbH, Laupheim, Germany.
Chemical analyses
Chemical analyses were performed by Toxikon Europe N.V. in Leuven, Belgium. Extraction of solid materials (containers, films, and resins) was done by refluxing the material in dichloromethane for eight hours. A ratio of 1-g sample per 10-mL solvent was used. In a separate experiment, it was verified that the dichloromethane reflux step did not induce oxidation of TBPP (data not shown). Sample preparation was performed by liquid-liquid extraction with dichloromethane at below pH 3, above pH 11, and with no pH adjustment prior to analysis of cell-culture medium incubated in single-use containers.
Quantification of TBPP, oxidized TBPP, and bDtBPP was performed with ultrahigh-performance liquid chromatography (UHPLC) with Exactive Orbitrap high-resolution accurate mass mass spectrometry (HRAM MS) (Thermo Scientific, Madison, WI, US). Mass spectrometry data were collected in both positive and negative mode using atmospheric pressure chemical ionization (APCI). The three compounds of interest were identified by exact mass data and compared with reference standards.
Cell-line maintenance
The CHO DG44 cell line was maintained in ActiCHO SM medium (Cytiva, Uppsala, Sweden) in 125-mL shake flasks on a rotary shaker at 100 rpm in a humidified incubator at 36.8 °C and 7.5% CO2. The cultures were split every third or fourth day to an initial viable cell density (VCD) of 0.3 × 106 cells/mL in 25 mL. Glutamine was added to the medium to a final concentration of 6 mM.
Cell growth-based film test method
Duplicate 2-L test containers were constructed from the film to be tested and 200-mL ActiCHO SM medium was added to each container (8 cm2 film/mL cell-culture medium). The containers were attached to a WAVE Bioreactor 20/50 EHT system (Cytiva) and inflated with air, after which inlet and outlet filters were clamped off to create a contained incubation system. ActiCHO SM media was incubated at 37 °C for three days, followed by four days of incubation at ambient temperature, for a total of seven days of incubation. The rocking parameters were 20 rpm and 6° angle during the incubation time. A bioreactor lid was used to avoid potential degradation of light-sensitive medium compounds. As a reference, ActiCHO SM cell-culture medium stored in the dark at 4 °C for seven days was used.
After seven days, the growth-promoting capacity of the respective incubated medium samples was assessed in triplicate 25-mL shake flask cultures. The cell inoculum was taken from maintenance cultures with a VCD of 3.5-5 × 106 cells/mL at passage 3 to 30 post-thaw. The inoculum was centrifuged at 400 × g for five minutes, after which the supernatant was discarded and the cell pellet suspended in 37 °C prewarmed test and control media to start cell concentration of 0.3 × 106 cells/mL. After three days, the VCD was determined using a cell counter (CASY TT, Schärfe System GmbH, Reutlingen, Germany). The number of population doublings (PD) was calculated according to Equation 1, where N0 is the VCD at culture start and N is the VCD at day three:

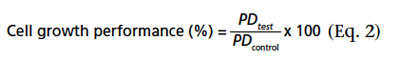
The mean coefficient of variation (CV) for the cell growth-based film test method was determined to be 3.2% with a range of 0.1-10.8% based on an assessment including six test containers, three operators, and three bioreactor systems (data not shown).
Dose-response experiment
The effect of bDtBPP on the CHO DG44 cell line was investigated in a dose-response experiment performed in shake-flask cultures. bDtBPP was dissolved in 10% dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS) buffer to make a stock solution with concentration 100 mg/L. ActiCHO SM cell-culture medium with 6 mM glutamine was spiked with bDtBPP from the stock solution to final concentrations of 0.05-2 mg bDtBPP/L. VCD and PD were assessed in triplicate 25-mL cultures, using the same methodology as described for the cell-growth test.
Results and Discussion
The aim of this study was to investigate how TBPP chemistry can be controlled during single-use container manufacturing by looking into the mechanisms of where and when the inhibitor compound bDtBPP is formed. An overview of a typical supply chain of a single-use container is schematically outlined in Figure 1.
![]()
Figure 1: Schematic overview of the supply chain segment, from bulk chemicals to end use.
Bulk chemicals, including TBPP, are compounded into resins and used for manufacturing of film for single-use containers; these containers are then sterilized and used in bioprocessing. To understand the origin of bDtBPP formation, species related to TBPP were tracked throughout the supply chain depicted in Figure 1. Levels of TBPP, oxidized TBPP, and bDtBPP (see structures in Figure 2) were measured in antioxidant standards, resins, films, sterilized containers, and finally, in cell-culture media that had been incubated in containers made from TBPP-containing film. For the antioxidant standard and all solid materials (resins, film sheets, and containers), the total amounts of the three compounds were measured. For the final step, mimicking end use, the amounts of the compounds leached into cell-culture medium were measured.
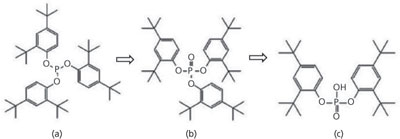
Figure 2: Chemical structures of (a) TBPP, (b) oxidized TBPP, and (c) bDtBPP.
For this study, five different lots of Solmed Infuflex 9101 PE-based multilayer film (Renolit) were selected from regular film production, spanning four years. These five film lots were denoted A1 to A5 and were manufactured from corresponding PE resin lots. In addition, one prototype film, denoted B, was manufactured from a prototype resin, also denoted B. Resins A and B are both PE resins with the same additive package and TBPP content. The two raw materials only differ in that resin B has a higher PE molecular weight and comonomer level.
Figure 3 summarizes the results of tracking TBPP-related species in samples from the entire supply chain, from bulk chemicals to end use. It shows that two steps are mainly responsible for ultimate generation of bDtBPP. The first is film extrusion, during which a large fraction of TBPP is converted into oxidized TBPP. The second is gamma sterilization, during which the remaining TBPP is converted to oxidized TBPP, which subsequently degrades into bDtBPP. Neither resin compounding nor storage of TBPP stock (fresh versus aged in Figure 3) contributes to oxidation of TBPP. Figure 3 also demonstrates that no bDtBPP is generated before the container irradiation step, consistent with the results reported by Hammond et al. (8). It is also evident from Figure 3 that higher amounts of oxidized TBPP in film led to higher amounts of bDtBPP in irradiated containers, with concomitantly higher levels of bDtBPP leached into cell-culture medium. Although based on limited data, leaching of oxidized TBPP from containers had no direct correlation to the amount of oxidized TBPP in the container. Additional data would be needed to verify/clarify this observation.

Figure 3: Distribution of tris(2,4-di-tert-butylphenyl)phosphite (TBPP), oxidized tris(2,4-di-tert- butylphenyl)phosphite (ox-TBPP) and bis(2,4-di-tert-butylphenyl)phosphate (bDtBPP) throughout the supply chain, from bulk chemicals to end use. The bars are normalized to the highest concentration measured within each group (TBPP standard/resin/film/container/media). Film A was manufactured at five different occasions (A1 to A5) and film B was manufactured once.
When comparing materials A and B in Figure 3, it can be seen that the extrusion step leads to 40-60% oxidation of TBPP with film A and 90% oxidation of TBPP with film B. PE resins A and B contain identical amounts of TBPP, so one would anticipate that the two films would liberate equal amounts of bDtBPP. The only difference is that resin B has a higher molecular weight and comonomer level and the corresponding change of the resin rheological properties required the film to be extruded under different conditions.
To further investigate the impact of the extrusion step on formation of oxidized TBPP, a simple two-factor study was executed. In this study, a single lot of A resin was used to produce one film within normal production settings (A) and three prototype films outside of normal production settings (A’). In addition, data from two normal production lots of A film were included (A1 and A2 in Figure 3). Because it is well known that oxidation of phosphite antioxidants such as TBPP is irreversibly triggered by heat (5), process parameters related to heating were studied. Selected parameters were extruder temperature (i.e., temperature during melting and processing of the resin), and output that reflects residence time and shear heating in the extruder (13). All combinations of low- and mid-settings (solid bars in Figure 4) are within the standard window of film A manufacturing settings. More extreme settings, outside of normal production window, are represented by the three high-setting corners (textured bars in Figure 4) and were used for producing the prototype films A’. The films A and A’ were analyzed for content of oxidized TBPP. In addition, cell-culture medium were incubated in gamma irradiated containers built from the films A and A’. Chemical analysis results are summarized in Figure 4.
Figure 4a shows that both extruder temperature and output had an effect on oxidization of TBPP during extrusion. By setting the two parameters past their standard manufacturing limits (Film A’), the level of oxidized TBPP increased more than two-fold (Figure 4a). Figure 4b shows that irradiated containers, built from the analogous films in Figure 4a, liberated substantially different amounts of bDtBPP into cell-culture medium. Containers built from films manufactured at higher settings leached higher amounts of bDtBPP. These results show that a single resin can be converted to films that contain substantially different amounts of leached bDtBPP, depending on how the resin is processed.

Figure 4: Effect of extrusion parameter settings on (a) resulting normalized levels of oxidized tris(2,4-di-tert-butylphenyl) phosphite in film manufactured according to the two-factor study, and (b) resulting levels of bis(2,4-di-tert-butylphenyl)phosphate (bDtBPP) leached into cell culture media incubated in containers made from these films. Solid bars are within standard manufacturing window (film A and regular production film lots A1 and A2). Textured bars are outside of standard manufacturing window (prototype film A’).
Given the apparent relationship revealed in Figure 4 between oxidized TBPP in film and bDtBPP leached into cell-culture medium, further investigations using the cell growth test method were performed. Single-use containers made from standard A films, prototype A’ films, and prototype B film were tested. Cell-culture medium was incubated in the containers, and cell growth of the CHO DG44 cell line was assessed by measuring VCD, expressed as PD relative to a control (PD %). The cell growth results are presented in Figure 5.
Oxidized TBPP is a precursor to bDtBPP and Figure 5a verifies that cell growth is least affected when the container is made from a film with low content of oxidized TBPP and is most adversely affected when the container is made from a film with high content of oxidized TBPP. Figure 5c shows that resulting cell growth, when using medium that has been incubated in bDtBPP-containing containers (Figure 5b), is very similar to the dose-response curve of Figure 5d where medium has been spiked with bDtBPP.

Figure 5: Incubation of cell culture medium in sterilized containers made from films A, A’, and B containing different levels of oxidized tris(2,4-di-tert-butylphenyl)phosphite (ox-TBPP). Correlation of cell growth to (a) concentration of ox-TBPP in films used for manufacturing of sterilized containers, (b) concentration of bis(2,4-di-tert-butylphenyl)phosphate (bDtBPP) in the sterilized containers, (c) concentration of bDtBPP leached from sterilized containers into cell-culture medium, and (d)concentration of bDtBPP spiked into cell-culture medium. (e) Correlation of cell growth with time post-irradiation to levels of bDtBPP leached from containers made from film B.
As shown in Figure 5e, cell-growth inhibition had a time-dependency post-gamma irradiation. Nonirradiated containers of prototype film B showed high cell growth. After gamma irradiation, bDtBPP was formed and leached at high levels, as resin B was extruded at a higher temperature because of its higher PE molecular weight. The high levels of leached bDtBPP caused severe cell growth inhibition. When retesting the irradiated containers after two years of real-time aging, the leached level of bDtBPP was decreased with a concomitant decrease in cell-growth inhibition. This phenomenon was observed for a range of films (data not shown). Thus, cell-growth inhibition induced by bDtBPP is of highest concern closely after SUS manufacturing and is decreased during product shelf life.
An important conclusion from Figure 5 is that cell-culture performance can be predicted already at the raw material level by measuring the content of oxidized TBPP in a well-characterized film. This finding can be used by SUS suppliers in raw material specifications to ensure consistent product performance in bioprocessing end use. There is no universal threshold value, however, for oxidized TBPP in films. The level of oxidized TBPP tolerable for sustaining high cell growth with a given film will be dependent of, for example, what layer of the film contains TBPP and what type of polymers comprise the film. Thus, each SUS-manufacturing process needs to be mapped for creation of a tailored manufacturing space that will generate a process where quality (i.e., high cell growth) is designed into the product, from raw material selection to raw material processing. This work can only be achieved by close and transparent collaborations throughout the supply chain.
Conclusions
Recent publications regarding the effects of TBPP degradation products on the quality of cell-culture performance have led to calls to manage TBPP levels in films. This case study has focused on how control of oxidative conditions during film manufacturing can be used for controlling the production of oxidized TBPP, the precursor of the cell growth inhibitor bDtBPP.
By quantifying the levels of TBPP, oxidized TBPP, and bDtBPP, from bulk chemicals to finished film, the extrusion step was identified as the major source of oxidation of TBPP during film manufacturing. The level of oxidation was demonstrated to be correlated to both the extruder temperature and the film output rate. It was shown that by varying the settings for temperature and output during extrusion of a single PE resin, the level of oxidized TBPP in the resulting film could be deliberately altered. As oxidized TBPP is a precursor of bDtBPP, gamma sterilized containers built from film containing higher levels of oxidized TBPP were shown to leach higher amounts of bDtBPP into the cell-culture medium, thereby showing a higher degree of cell growth inhibition. Thus, this case study demonstrates that a single resin with a fixed additive package can be converted into a film that either sustains or inhibits cell growth, depending on the film manufacturing conditions used. Consequently, the chemistry of the base polymer itself can be as equally as important for cell growth as the additive package. Base polymer properties, such as molecular weight, will determine polymer-melting temperature. Polymer melting temperature is directly correlated to the temperature needed for processing of a resin during extrusion, which in turn, controls the formation of oxidized TBPP. Based on the identified correlation, cell-culture performance of gamma-sterilized containers could be predicted by measuring the level of oxidized TBPP in the film prior to container manufacturing.
These results generate further insight into ways to control film and container production as part of SUS manufacturing. Having solid methods in place for testing critical quality attributes of the end product is key for critical raw materials management. By establishing a correlation between input parameters (raw material chemistry and extrusion settings) and output parameters (leached level of bDtBPP and cell growth), both quality and control are designed into the manufacturing process. This correlation can be used by SUS suppliers in raw-material specifications to assure consistent product performance in bioprocessing end use.
Acknowledgements
The authors thank Matt Hammond (Amgen Inc., CA, US) for kindly providing the compound bDtBPP for the cell line dose-response titration experiment, Yvette Klingberg, Anita Vitina, Åsa Lundström, and Andreas Andersson (Cytiva, Uppsala, Sweden) for cell-culture work, Paul Michael (Cytiva, Cardiff, UK) for cell-culture method data modelling, and Michael Miller (Cytiva, Westborough, US) for container manufacturing. The authors thank Theo Vriend, Edmond Mulkens, Lodewijk Berkenbosch, Peter Karsten, Peter Robben, Ivo Kooijman, and Ellen Repkes (Renolit) for many fruitful discussions.
References
1. R.A. Stock, American Pharmaceutical Review (Oct. 1, 2010).
2. T. Kingsbury, BioProcess Int. 12 (4) 18-21 (2014).
3. H.F. Giles, E.M. Mount, and J.R. Wagner, The Definitive Processing Guide and Handbook, Part 6: Coextrusion, Coextrusion Applications (William Andrew Inc., NY, NY, 2005), pp. 372-389.
4. M.A. Ruberto, “Polymers and additives used in fabrication of disposable bioprocess equipment” in supplement to BioProcess Int. (4) s36-41 (2010).
5. Zweifel et al., Plastics Additives Handbook, (Carl Hanser Publishers, Verlag, Munich, 6th ed., 2008), pp. 13.
6. T. Czvikovszky, “Degradation effects in polymers,” in Advances of Radiation Chemistry of Polymers, pp. 91-101 (2004).
7. E. Lokensgard, Industrial Plastics: Theory and Applications (Delmar Cengage Learning, Canada, 5th ed., 2009), pp. 106.
8. M. Hammond, H. Nunn, and G. Rogers et al., PDA J. Pharm. Sci. Tech. 67 (2) 123-134 (2013).
9. J. Wood, E. Mahajan, and M. Shiratori, Biotechnol. Prog. 29 (6) 1535-1549 (2013).
10. R. Eibl, N. Steiger, and C. Fritz et al., “Standardized cell culture test for the early identification of critical films,” in the Recommendation for Leachables Studies whitepaper, Dechema pp. 1-24 (2014)
11. M. Hammond, L. Marghitoiu, and H. Lee et al., Biotechnol. Progr. 30 (2) 332-337 (2014).
12. J. Martin, BioPharm Intl. 27 (2) pp. 16, 18, 48.
13. H.F. Mark, Encyclopedia of Polymer Science and Technology (John Wiley, Hoboken, NJ, vol. 2, 2003), pp. 49
About the Authors
Sara Ullsten, PhD is bioprocess project manager; Eva Lindskog, PhD, is upstream marketing segment leader; Eva Blanck is research engineer; Shujian Yi, PhD, is senior polymer scientist; and Jeffrey Carter, PhD, is strategic regulatory services leader, all at Cytiva. Pokon Ganguli is R&D manager at Renolit Nederland B.V., and Hernan Parma is business development manager at American Renolit Corp.
Article Details
BioPharm International
Vol. 28, Issue 1
Pages: 22–29
Citation: When referring to this article, please cite it as S. Ullsten et al., "Implementation of Raw Material Control Strategies in the Manufacture of Single-Use Bioprocessing Containers," BioPharm International 28 (1) 2015.