 Innovative approaches, including ready-to-use materials and in-line dilution, can significantly streamline overall bioprocessing operations.
Innovative approaches, including ready-to-use materials and in-line dilution, can significantly streamline overall bioprocessing operations.
By: Pranav Vengsarkar and Nandu Deorkar
It is common for biologics manufacturing processes to require hundreds of raw materials, ranging from media and media supplements, buffers, and salts to other process chemicals. Buffers and salts are typically the largest constituents by volume used in the downstream processing steps for manufacturing most biopharmaceutical products. A typical monoclonal antibody (mAb) process may require 2000 L of a buffer and more than 100,000 L per batch based on titer and bioreactor volume (1, 2). As a result, the variety of buffer solutions used across a biopharma manufacturing facility requires flexible, cost-efficient infrastructure and on-time delivery to meet process demand.
At the same time, the growing use of single-use systems offers many advantages in managing raw materials used in bioprocessing. Single-use technologies add flexibility, reduce capital requirements, and improve ease-of-use in biopharma manufacturing operations. Solid or hydrated buffers and salts that come ready-to-dispense in single-use bags and buffer preparation using single-use systems, reactors, and in-line dilution can both help eliminate or reduce weigh and dispense operations.
The modularity of single-use systems allows site-specific and process-specific solutions to be implemented quickly without requiring significant capital expenditure. Single-use raw material delivery systems, in powder or liquid form, can help reduce the risk of contamination and minimize the need for frequent cleaning steps. Raw material quality control (QC) testing can also be reduced or eliminated by implementing single-use technologies, saving time from reduced process steps as well as labor costs.
The increase in titers and the trend toward continuous biomanufacturing will lead to increased demand in buffer volumes and prep times. To address this gap, strategies for improving mAb manufacturing productivity by optimizing powder and liquid buffer and media prep process flow are worth considering. When combined with innovative new technologies such as in-line dilution, single-use powder and liquid formulations can help reduce facility footprint, labor hours, and the overall cost of goods.
Powder-handling process improvements
A typical powder-handling process at a biomanufacturing site starts with raw material testing (of each lot or identity tests). Following the raw material testing process, powders are weighed and dispensed into a separate container (e.g., intermediate bulk container [IBC], bags), and kitting and buffer component storage are done in a storage area. This process requires proper recording and tracking of buffer component kits until the subdivided materials can be dispensed into a single-use or stainless-steel mixing vessel according to the specific amount needed for a given process.
Most of these material handling processes take place in a classified environment as a segregated operation to reduce cross-contamination and particulate risks (2). This operation can take anywhere from 25 to 32 working hours per material handled to produce one batch of a buffer. Along with the time involved, there are many other challenges with this process (3):
- Intrusive physical sampling of powder raw materials requires rapid material usage post-sampling to comply with regulatory guidance.
- The physical properties of powders require extensive cleaning of classified prep areas to prevent cross-contamination.
- Many powders have properties that cause them to clump or cause static holdup, increasing the labor required per batch and the risk of potential safety incidents.
- Solid agglomerates (or material clumps) can cause damage to single-use or stainless-steel tanks.

The use of pre-weighed and kitted materials can help address some of these issues (Figure 1). There are several ways to implement a new process depending on the buffer/salt component, buffer volume requirements, production schedule, and desired flexibility (Table I). One approach uses materials in single-use, ready-to-dispense packaging supplied at custom weights; the other uses modular packaging provided at standard weights.
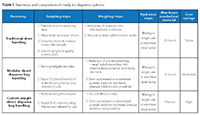
Manufacturing sites dedicated to producing a limited number of products following a stable schedule are likely to have greater success using the custom weight approach. A biopharma manufacturer could request the supplier to provide material at a precise weight (e.g., 51.223 kg). With this approach, the manufacturer can quickly dispense the material to make a buffer of a known concentration. This method also eliminates any on-site powder kitting process steps while reducing hours required per buffer batch to almost a third of the typical process.
An equally effective approach lets a manufacturer make efficient use of a supplier’s standard, modular-weight system. Products can be provided in predefined weights-100 kg, 25 kg, or 1 kg, for example-for each buffer or salt component in a ready-to-dispense format. This method, ideal for sites that make several products with frequent schedule changes, offers more flexibility to make buffers on-site. A small subdivision process is still required to support the precise weighing of solids in the sub-kilogram scale. Overall, the modular weight system can reduce the working hours needed per buffer significantly as compared to the traditional approach.
Pre-weighing processes can be used with single-use powder delivery packaging. This packaging is filled in a controlled environment with traceable trusted weight stickers, ensuring accurate weights delivered into a buffer tank. This packaging method can improve overall process efficiency, regulatory compliance, and buffer quality.
A manufacturer should consider the powdered materials used in single-use delivery systems, examining the materials’ properties, including:
- Chemical nature of solid (anhydrous, hydrated salt, hygroscopicity)
- Physical nature of solid (pellet, granule, fine powder)
- Compressibility and flowability (bulk density, Hausner ratio)
- Safety (hazardous solids, minimum ignition energy for fine powders)
- Dissolution rates (affects the speed of dispensing and hence bag port size)
- Others (static nature, stability, compatibility with plastics).
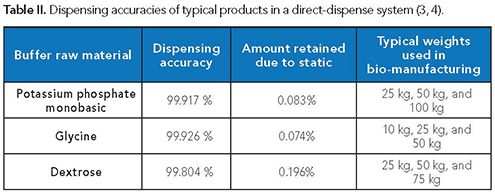
Field studies gathered where the packaging was in use assess the accuracy of the product delivered into the production systems. Data for such cases indicate a dispensing tolerance close to 100% for some common raw materials (Table II).
A powder delivery system that supports non-contact, rapid identification methodologies, such as Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy, can streamline the incoming testing of the powders and reduce the risk of contamination (Figure 2). The availability of a side sample of the material can further help streamline process steps, mitigate risk, and ensure all quality and regulatory requirements are met.

Advances in liquid handling processes
The adoption of premade liquid buffers and buffer concentrates into biomanufacturing has been limited up to this point to simple cleaning buffers, such as caustic solutions and other hazardous products that have environmental health and safety (EH&S) concerns, such as hydrochloric acid. High shipping costs may have limited the ability to extend the use of this technology with other materials. Improvements in equipment and single-use system technologies, however, have made this option more appealing.
Hydrated buffers and salts in biomanufacturing can help enable continuous processing while eliminating issues related to powder handling. Sterile-filtered solutions of complex 1X buffers or buffer concentrates are now more feasible due to the availability of single-use containers ranging in volumes from 200 L to 1000 L. Advances related to in-line dilution systems using multicomponent concentrates (Figure 3) and in-line conditioning systems/buffer stock blending systems using single-component concentrates have also made the use of these buffer concentrates economically viable.

Recently, the BioPhorum Operations Group (BPOG) conducted an economic analysis of the use of liquid handling systems and buffer concentrates (1, 5). The report highlights operational savings from using concentrated stock solutions along with a buffer stock blending system. Savings vary based on the size of the facility and the capital cost required. Nevertheless, the overall technology can generate significant process improvements in downstream buffer preparation.
Deploying liquid handling systems can require planning and analysis to assess suitability for a specific operation because liquid raw materials have several challenges compared to powders. Several parameters are critical to consider when developing buffer concentrates as raw materials and when using them, such as:
- Stability of essential product parameters over time (pH, conductivity, assay; critical for chromatography)
- Bioburden and endotoxin (critical for growth-promoting solutions)
- Densities and viscosities of concentrates (critical to ensure accurate flow and pump sizing)
- Safety (hazardous solutions can pose a challenge at large scales)
- Corrosive nature of solutions (can affect extractable and leachable profiles of single-use systems and tanks)
- Freezing points and temperature stability of solutions (necessary to mitigate issues post-shipping).
A simple failure mode and effect analysis (FMEA) can guide such evaluation and implementation options. Stability studies on these buffer concentrates following International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Q7 guidelines (6), extractable and leachable profiles using BPOG guidelines on single-use components, and bioburden and endotoxin specifications on validated processes to manufacture these concentrates are some of the necessary critical quality items.
They can ensure compliance with quality and regulatory requirements and proper functioning of the processes. Harmonization between vendor test methods and raw material data management will enable the real-time release of buffer stocks and reduce the risk of noncompliance. Reduced in-house testing will also allow for increased on-site effectiveness.
The added costs will be for the storage of these premade stocks and their management. In some cases, buffer vendors are addressing these storage and management issues by also offering local current good distribution practices storage facilities, providing additional flexibility and de-risking the supply chain.
Conclusion
Buffer and media preparation are vital components of the mAb manufacturing process, requiring significant efforts and time. Innovative approaches comprising of pre-weighed, ready-to-dispense solid packaging systems, ready-to-use diluted buffers, and ready-to-use buffer concentrates with in-line dilution or conditioning systems can significantly help streamline bioprocessing operations.
Improving the efficiency and productivity of buffer preparation and delivery operations can significantly improve the overall performance of mAb manufacturing. The most suitable approach will depend on the manufacturing operation’s type, size, and required flexibility. Continuing innovations in single-use technologies, process analytical technologies, and in-line dilution will offer the potential for further significant benefits in the future.
Acknowledgments
The authors would like to thank the following people at Avantor for contributing to the data used in this article: Jungmin Oh, Tom Lee, Calvin Cheah, and Andrew Kalinovich.
References
1. BioPhorum Operations Group, “An Economic Evaluation of Buffer Preparation Philosophies for the Biopharmaceutical Industry,” BioPhorum.com, December 2019.
2. N. Deorkar, “Enhancing Cleanroom QC and Efficiency Through Direct Dispense Technology,” CleanroomTechnology.com, Oct. 11, 2017.
3. Private communications/internal data.
4. W. Hesselink, et al., “Implementation of Single-Use Powder Delivery Systems in Continuous Bio-Manufacturing Processes: A Case Study,” PharmaiQ.com, accessed June 5, 2020.
5. BioPhorum Operations Group, “NIIMBL-Biophorum Buffer Stock Blending System: a More Advanced Concept for Buffer Manufacturing,” BioPhorum.com, December 2019.
6. ICH, Q7 Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients, Step 4 version (ICH, Nov. 10, 2000).