Production of Influenza virus using ReadyToProcess single-use equipment
To meet the challenges in today’s vaccine manufacturing, a case study was conducted of a scaled-up vaccine production based solely on single-use equipment. Whole live influenza virus was used as model system. A summary of the production scale-up from laboratory scale is presented 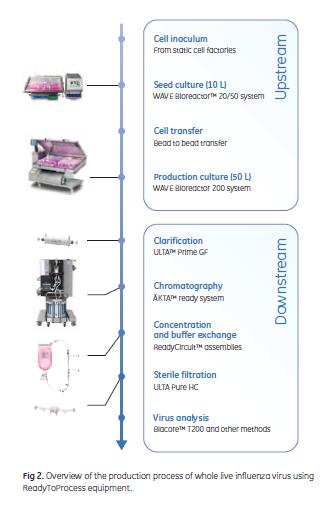 in this white paper. More detailed descriptions of the individual upstream and downstream processes are presented in separate application notes (4, 5).
in this white paper. More detailed descriptions of the individual upstream and downstream processes are presented in separate application notes (4, 5).
Figure 2 gives an overview of the upstream and downstream processes including cell expansion, influenza propagation, purification, and analysis.
Upstream processing
Adherent Vero cells were expanded in static cell factories to generate inoculum for 10 L seed cultures. The cells in the 10 L cultures were grown on Cytodex 1 microcarriers (3 g/L) in the WAVE Bioreactor 20/50 system. The cells were grown to a cell concentration of approximately 3 × 106 cells/mL. The cells were subsequently detached from the microcarriers with trypsin and used to seed a 50 L microcarrier culture in the WAVE Bioreactor 200 system. The microcarrier concentration was kept constant. Sufficient cell recoveries were obtained for a split ratio of 1:5 during scale up from 10 L to 50 L working volume.
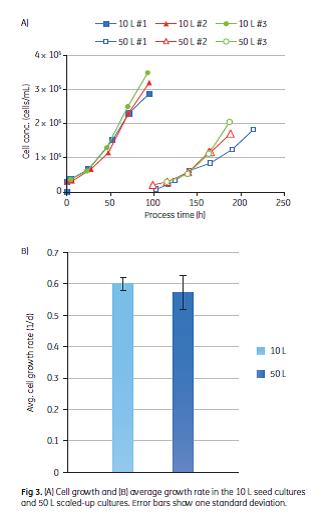
A common challenge upon cell transfer is the lag phase, during which the cells are adjusting to the new culture medium and little or no increase in cell number is observed. In the 50 L cultivations, the cells reattached and started to grow on the new microcarriers without lag phase. The cell growth rate was shown to be similar in both the 10 L and 50 L cultures in three consecutive batches (Fig 3).
 In the 50 L cultures, cells were grown to a cell density of approximately 2 × 106 cells/mL. The cells were infected with influenza virus strain A/H1N1/Solomon Island during the exponential growth phase at a multiplicity of infection of 4 × 10-3 and a trypsin concentration of 2 mg/L. Samples were taken daily during the infection phase. As shown in Figure 4, the infectious and total amount of virus particles initially increased quickly and then remained at a constant level until time of harvest, when the amount of virus particles was determined to be approximately 109 virus particles/mL. The haemagglutinin (HA) concentration increased steadily throughout the infection phase and was approximately 12 μg/mL at time of harvest. Similar virus titers and HA concentrations were measured in the 10 L culture (data not shown) as in the 50 L culture (Fig 4).
In the 50 L cultures, cells were grown to a cell density of approximately 2 × 106 cells/mL. The cells were infected with influenza virus strain A/H1N1/Solomon Island during the exponential growth phase at a multiplicity of infection of 4 × 10-3 and a trypsin concentration of 2 mg/L. Samples were taken daily during the infection phase. As shown in Figure 4, the infectious and total amount of virus particles initially increased quickly and then remained at a constant level until time of harvest, when the amount of virus particles was determined to be approximately 109 virus particles/mL. The haemagglutinin (HA) concentration increased steadily throughout the infection phase and was approximately 12 μg/mL at time of harvest. Similar virus titers and HA concentrations were measured in the 10 L culture (data not shown) as in the 50 L culture (Fig 4).
A more detailed description of the cell culturing and virus infection and harvest is given in the application note 29-0435-48 (4).