Program Steps Defined
TPP/QTPP. FDA's 2007 draft guidance for industry defines TPP as a format for a summary of a drug development program described in terms of labeling concept (1). QTPP is defined in ICH Q8 (R2) as "a prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure the desired quality, taking into account safety and efficacy of the drug product" (2).
Assessment of knowledge. For each product under development, an initial assessment of available knowledge regarding the product and its function is assessed using various methods such as previous experience, literature information, consultants, and knowledge gap analysis. Efforts will be made to close the knowledge gap. This process helps in identifying the critical quality attributes (CQA) and is a joint effort between the client and the CMO.
CQA. CQAs are defined through execution of a risk assessment, which evaluates the impact of each product attribute on the safety and efficacy of the product. This is an iterative exercise conducted by key stakeholders from the product development team (CMO and client). Input from the clinical experts is critical for successful definition of CQAs.
Critical process parameters (CPP). Once CQAs are established, process parameters from each unit operation are evaluated to determine potential contribution or impact on those CQAs.
Design of experiments (DOE) and design space. Based upon an initial risk assessment and process evaluation, a structured experimental plan, designed for statistical analysis, begins. This analysis provides an understanding of how the process parameters and their interactions impact CQAs. Each CQA then serves as a dimension in the establishment of a multidimensional design space that provides a full understanding of process impact on product quality.
Process control strategy (PCS). A product developed using QbD principles will rely on manufacturing controls based on what is known to be crucial to the process. The PCS package results in a well-defined process with criticality understood and all process ranges justified with statistically significant data.
Continuous process verification (CPV). A good CPV program assures the process is under control and that any process drift in performance is detectable and can be proactively addressed.
Breaking Down the Critical Parts of Criticality
The first step begins with the generation of a target product profile (TPP) with safety and efficacy as central components. The main focus of QbD is to ensure that the elements of quality are incorporated into the design of the process and that the process consistently yields a safe and efficacious product. Product understanding with regard to safety and efficacy is limited at the beginning of the process unless the molecule in question belongs to a class of products already in use (e.g., as would be the case in the development of a biosimilar). Initial lack of information regarding the manufacturing process can be reduced to a certain extent based on experience, platform processes, and risk assessment exercises. The advantage of the CMO in this environment is the large breadth of experience and comparability tools available for the many diverse products using similar process steps.
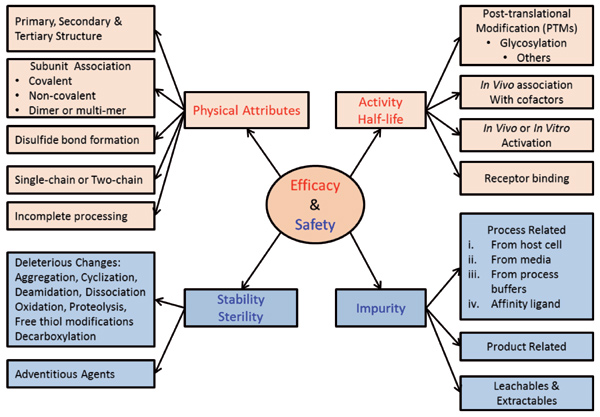
The first customized tool used in a QbD-based process development is the CQA risk assessment. CQAs are product attributes that have the most impact on the efficacy and safety of the product. At the beginning, this will be a preliminary list based upon the QTPP and might include a large number of attributes. As the product knowledge and the process knowledge increase, the number of CQAs might reduce. A tool shown in Figure 2 is used to initiate CQA determination from the TPP/QTPP. Information from literature, previous experience, subject matter experts, and consultants are used to generate the list of potential CQAs. A customized risk assessment tool (separate from the tool in Figure 2) takes into account "uncertainty" and "impact" of each CQA on safety and efficacy of the product. The preliminary list of CQAs helps prioritize which attributes to study further to understand and reduce uncertainty.