 Principles and methodologies for biopharmaceutical development and manufacturing are well established today. However, the increased molecular diversity and drive for higher productivity brings new challenges for process developers. One of these challenges is how to get deeper understanding for sources of variability and set mitigation strategies.
Principles and methodologies for biopharmaceutical development and manufacturing are well established today. However, the increased molecular diversity and drive for higher productivity brings new challenges for process developers. One of these challenges is how to get deeper understanding for sources of variability and set mitigation strategies.
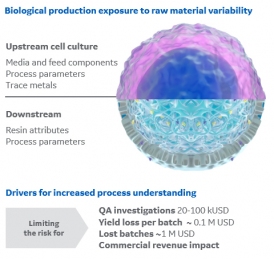
This article shares some insights into how raw material variability is a potential source for process variation. The focus lies on chromatography resins.
Click here to read more.