 Downstream processing involves the recovery and purification of protein therapeutics for biopharmaceuticals, vaccines, and other biologics. It involves the removal of insoluble cell debris and particulates; product isolation through extraction, adsorption, ultrafiltration, or precipitation; product purification through affinity chromatography, crystallization, or precipitation; and product polishing and virus removal.
Downstream processing involves the recovery and purification of protein therapeutics for biopharmaceuticals, vaccines, and other biologics. It involves the removal of insoluble cell debris and particulates; product isolation through extraction, adsorption, ultrafiltration, or precipitation; product purification through affinity chromatography, crystallization, or precipitation; and product polishing and virus removal.
Downstream processing (DSP) has seen significant challenges over the past few years. Improved upstream bioprocessing has created increasingly higher product titres and resulted in purification bottle necks. In addition, the recent increase in upstream single-use technologies has created pressures on DSP operations to keep up.
DSP remains one of the most crucial and talked-about areas of biopharmaceutical manufacturing. Indeed, a survey conducted in late 2012 among 450 global subject matter experts and senior participants who make up BioPlan’s Biotechnology Industry Council confirmed that a leading 24% of bioprocessing decision-makers indicated that downstream processing would be the biggest trend in 2013 (1).
Although much of the discussion surrounding downstream purification concerns resolving or avoid short-term capacity constraints, the long-term implications to the industry are more crucial and significant in terms of improving overall bioprocessing, increasing the number of world-class bioprocessing facilities, and lowering overall costs of bioprocessing. This more strategic view is recognized by both end-users and suppliers and technology innovators. And in BioPlan’s recently released 10th Annual Report and Survey of Biopharmaceutical Manufacturing (2), it is found that DSP is one of the top areas in which both industry suppliers and end-users are developing and evaluating new technologies and other options for improving their downstream processes.
Many of the same challenges that have plagued the industry over the past few years continue:
• Cost of chromatography materials
• Lack of single-use (disposable) options
• Cost of membranes
• Cleaning and validation costs
• Time for operations.
In addition, while many firms would like to avoid the high cost of Protein A affinity resin, most find it difficult to make such changes to their processes, and their reluctance is compounded by the fact that there remain few feasible alternatives that have been clearly proven at a large scale.
Downstream’s Impact on Overall Capacity
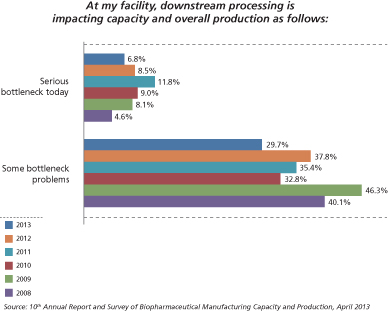
DSP clearly has strategic manufacturing implications. Data from BioPlan’s Annual Report indicates that about three in four facilities are experiencing or expecting at least minor capacity bottlenecks as a result of downstream processes (2). The good news is that the severity of the constraints appears to be leveling off. Specifically, 6.8% (vs. 8.5% last year) of respondents agreed that their facility was experiencing “Serious bottlenecks today,” and a lower proportion, 29.7% (37.8% last year), reported that they were experiencing “Some bottleneck problems.” Therefore, indications of more serious bottlenecks appear to be on the decline (see Figure 1). This is consistent with BioPlan’s research indicating implementation of specific, incremental improvements in downstream unit operations.
Anecdotal responses to the survey align with the data. This year, relatively few respondents commented on the negative impact of downstream steps. Representative comments included:
• Bottleneck at Protein-A chromatography
• Bottlenecks could occur in the next five years
• Clarification before column purification is a bottleneck problem
• New processes being introduced could create downstream-related delays at process development stage.
By contrast, prior year comments included more detail and more indication of problems:
• Downstream still is not keeping up with increasing titers.
• Expression systems are producing 5 times what they were 10 years ago.
• Current downstream/purification areas were not designed to handle this increase in production.
• Buffer capacity, column size, UF membrane area are all problems.
• Primary clarification is a major bottleneck.
• We need multiple trains.
• We need better affinity columns (protein A).
• The problem is buffer capacity and need for in-line dilution.