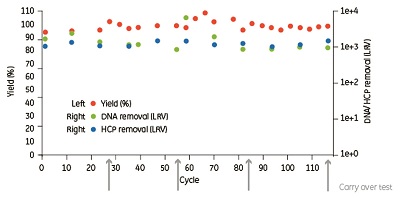
The results show that the step yield was consistently over 95%, and high log-reduction factors of host-cell proteins (HCP) and DNA were achieved (see Figure 1). In this study, carryover was evaluated each 28th cycle and was found to be less than 0.1% (i.e., after cycle 28, 56, 84, and 112). The lifetime study with mAb-containing feedstock demonstrates that the product quality, DBC, and yield with Resin 3 were stable for more than 100 purification cycles. No increase in pressure was observed during the study.
Cost performance
Cost performance is dependent on product amount produced per year, batch size, column size, and acceptable process time. In this study, the cost of Protein A-based production was calculated titer by titer by the use of conventional resins (Resin 1, which is Cytiva’s rProtein A Sepharose 4 Fast Flow, and Resin 2) and Resin 3 prototype resin. The calculations are based on an annual mAb production amount of 500 kg and bioreactor size of 10,000 L for 1 g/L titer or 5000 L for 3 g/L and 5 g/L titers. Column diameter was estimated by considering a process time within 10 to 15 h.
The total purification cost per kilogram of produced antibody from 1 g/L and 3 g/L titers, including chemicals and water, using Resin 1 ($13,000 and $14,000 respectively) can be reduced by 29% and 46% respectively by using Resin 2 instead, as shown in Figure 2.
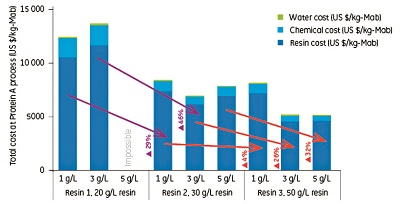
With Resin 3, in the case of a titer level of 3 g/L, the authors found that the overall purification cost can be even further reduced by 26%. With a lower titer level of 1 g/L, however, the decrease was only 4%.
Under the selected conditions, Resin 1 was not suitable for purification of antibody from a 5 g/L titer. The purification cost per kilogram of produced antibody from a 5 g/L titer using Resin
2 (7000 USD) can be reduced by 32% by using Resin 3 (see Figure 2).
These results show that the use of Resin 3 in purification of antibodies from high-titer feeds significantly improves process economy (see Figure 2).
Summary
These data demonstrate that the Resin 3 prototype has high capacity and reusability with stable step yield and impurity clearance (e.g., DNA, HCP) for more than 100 cycles. The engineered Protein A ligand allows for the use of rigorous and cost-effective CIP and sanitization protocols based on NaOH. Furthermore, the ligand is protease stable, which leads to lower ligand leakage, and the highly cross-linked agarose matrix allows for high flow velocities at production scale.
In conclusion, process economy can be significantly improved by the use of Resin 3 in purification of monoclonal antibodies from high-titer cell culture supernatants.
Acknowledgements
The authors wish to thank Cytiva (Uppsala, Sweden) for providing Resin 3 prototype resin.
References
1. G. Köhler and C. Milstein, Nature 256 (5517) 495-497 (1975).
2. S. Kozlowski and P. Swann, Adv. Drug Deliv. Rev. 58 (5-6) 707- 722 (2006).
3. P.A. Scolnik, mAbs 1 (2), 179-184 (2009).
4. S. Aggarwal, Nat. Biotechnol. 29 (12) 1083-1089 (2011).
5. B. Kelley, mAbs 1 (5), 443-452 (2009).
6. S. Lofdahl, et al., Proc. Natl. Acad. Sci. USA 80 (3) 697-701 (1983).
7. D. Colbert, et al., J. Biol. Response Mod. 3 (3) 255-259 (1984).
8. Cytiva, “Dynamic binding capacity study on MabSelect SuRe LX for capturing high-titer monoclonal antibodies,” Application Note, 28-9875-25, Edition AA.