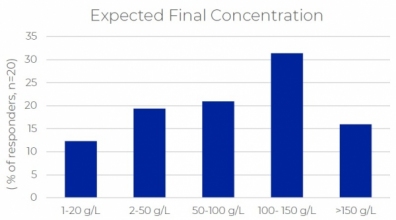
The growth in the formulation of drugs suitable for delivery by subcutaneous (Sub-C) injection highlights manufacturing challenges associated with the higher concentration of active ingredients and formulation components required. Manufacturing unit operations that are directly impacted by higher concentration formulations range from ultrafiltration to sterile filtration, filling, mixing and storage. Solutions that maintain quality, maximize process yield and product recovery are available and can be introduced to adapt an existing manufacturing platform to the new process requirements.