Efficient and Scalable Buffer Preparation Blog Post
This article describes how to ensure a scalable process for buffer management from process…

This article describes how to ensure a scalable process for buffer management from process…
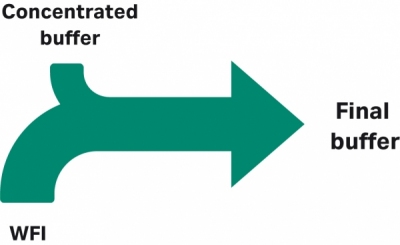
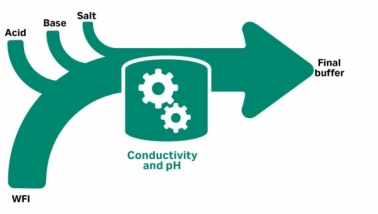
A typical bioprocess workflow requires many buffers and process liquids in large volumes…
It is common for biologics manufacturing processes to require hundreds of raw materials…

Among the various downstream processing unit operations that are used for purification of biotech…
Development of new modalities of biotherapeutic molecules will rely on manufacturing, regulatory, and collaborative support.

Outsourcing of analytics to CROs can help drug developers realize these goals in a cost-effective manner.


Single-use (SU) technologies increasingly find use today at all phases of the drug development cycle from preclinical